Growing and moving: How interactions between neuronal migration and outgrowth shape network architecture
Neurons are sociable cells that, on the long run, die in isolation. During development, they therefore grow out cellular processes, termed neurites, to establish synaptic connections with other neurons. Once they receive sufficient or too much synaptic input, however, they stop growing or shrink. By this, neurons avoid long-term over-excitation. It is widely assumed among researchers that neuronal growth is hereby controlled to stabilize neuronal activity at a specific target level.
Yet, to increase the probability of connections, neurons cannot only grow out their neurites but are also able to migrate towards other neurons. “In computer simulations we show that migration and neurite outgrowth may interact to shape specific mesoscale network architectures” says Samora Okujeni. The interaction regulates the relation between local connectivity within clusters and long-range inter-cluster connectivity and thereby the degree of network modularity. “This, in turn, influences the generation and spatiotemporal patterns of spontaneous activity.” Such interdependencies may be crucial for the proper development of the cortex.
The scientists tested the model predictions experimentally by investigating how cell migration, neurite outgrowth and activity interact in developing networks of cultured rat cortical neurons. To modulate cell migration in these networks they manipulated an enzyme that is centrally involved in the regulation of the neuronal cytoskeleton. As in their simulations, cell migration and clustering likewise promoted modular connectivity in vitro.
Yet, in addition, clustering promoted activity generation and led to higher activity levels. This was inconsistent with the assumed regulation of growth to establish a common target activity level. The scientists could resolve this discrepancy: “Cytoskeletal dynamics are not directly controlled by action potential activity but indirectly through an associated calcium influx that influences the balance between growth and degradation.” Okujeni explains. “Modularity increased the overall rate of action potentials but decreased their synchronization across the network that effectively determines the calcium influx per action potential. Given this dependence, we estimated that all network structures attain a similar target level of calcium influx during development.”
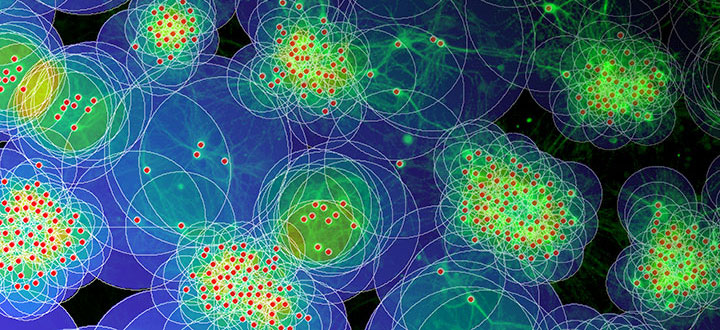
Figure Legend
Based on in vitro studies and computational modeling, neuroscientists show how neuronal outgrowth and migration interact in shaping network architecture and the degree of modularity in mature networks. Photo: Samora Okujeni | Bernstein Center Freiburg
Original Publication
Okujeni S and Egert U (2019). Self-organization of Modular Network Architecture by Activity-dependent Neuronal Migration and Outgrowth. eLife 2019;8:e47996
doi: 10.7554/eLife.47996
Further Information
|
Publication |
Okujeni S and Egert U (2019). Inhomogeneities in Network Structure and Excitability Govern Initiation and Propagation of Spontaneous Burst Activity. Front. Neurosci. 13:543.
doi: 10.3389/fnins.2019.00543 |
|
Publication |
Okujeni S, Kandler S, Egert U (2017). Mesoscale Architecture Shapes Initiation and Richness of Spontaneous Network Activity. J Neurosci 37:3972–3987.
doi:10.1523/JNEUROSCI.2552-16.2017 |
|
Press Release |
Press Release University of Freiburg (DE) |
Video on Media Portal University of Freiburg
Contact
Dr. Samora Okujeni
+49 (0)761/203 - 7523
E-mail: samora.okujeni@imtek.uni-freiburg.de
Prof. Dr. Ulrich Egert
Tel.: +49 (0)761 203-7524
E-mail: egert@imtek.uni-freiburg.de
University of Freiburg
Bernstein Center Freiburg
Hansastr. 9a
79104 Freiburg
and
Faculty of Engineering
Biomicrotechnology, Dept. of Microsystems Engineering
Georges-Köhler-Allee 102
79110 Freiburg